A Colorimetric Chemodosimeter for Pd(II): A Method for Detecting Residual Palladium in Cross-Coupling Reactions. - Abstract - Europe PMC
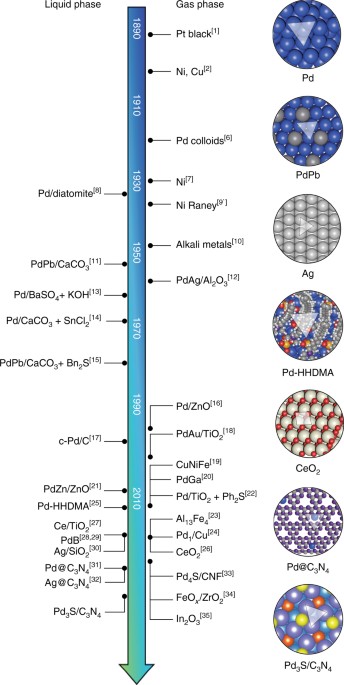
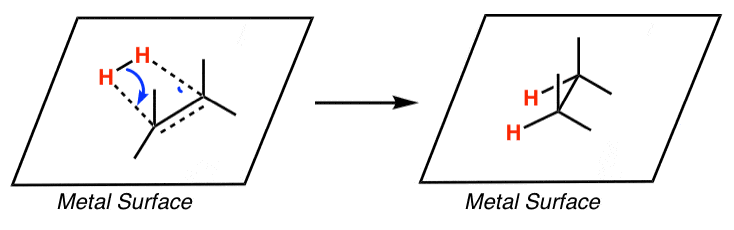
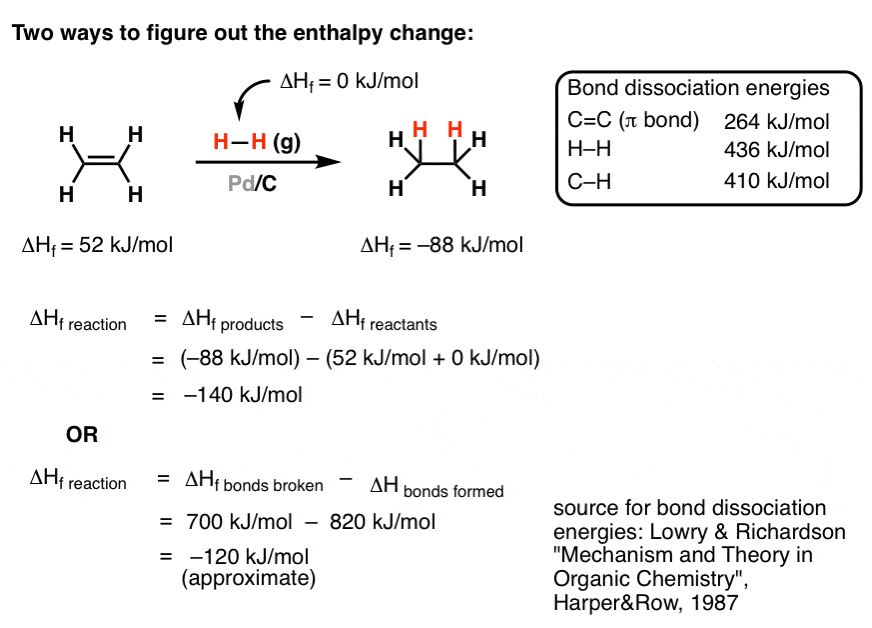
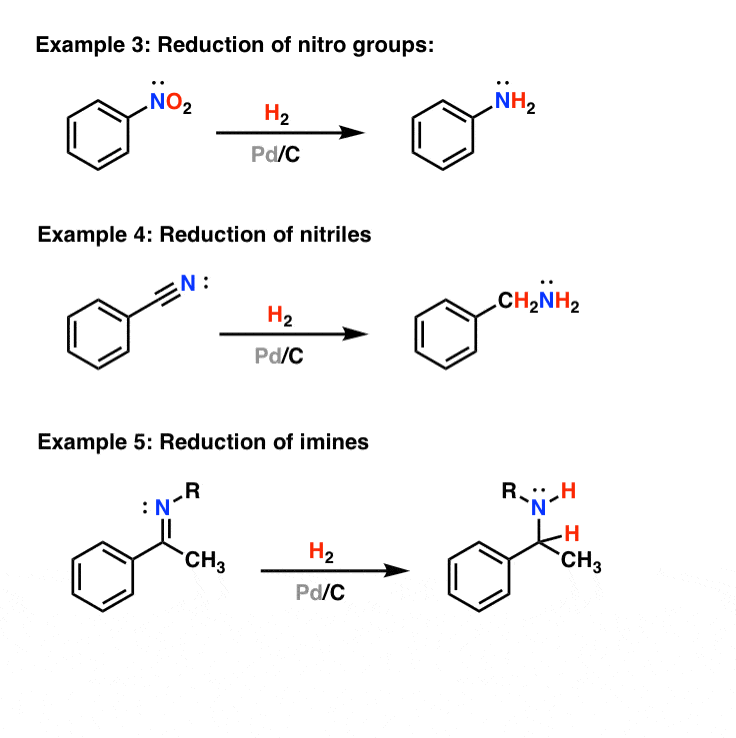
Selective ensembles in supported palladium sulfide nanoparticles for alkyne semi-hydrogenation | Nature Communications
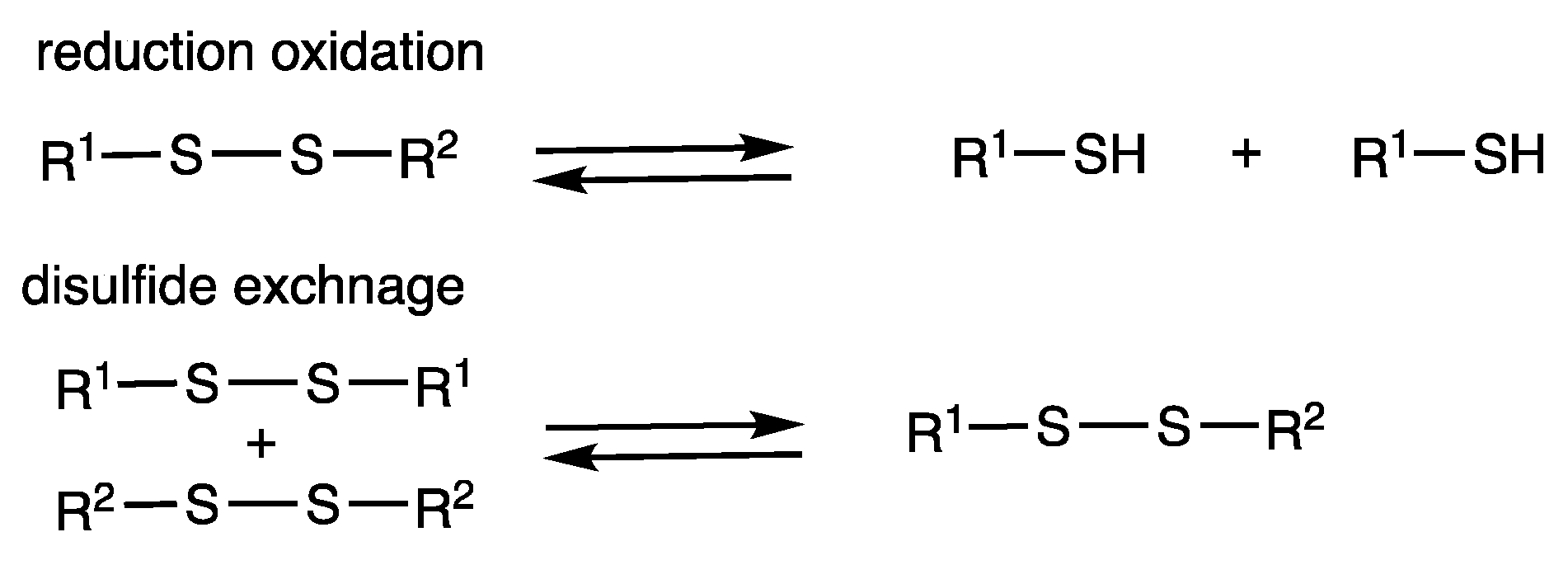
Molecules | Free Full-Text | Rhodium-Catalyzed Synthesis of Organosulfur Compounds Involving S-S Bond Cleavage of Disulfides and Sulfur | HTML

Bond-Forming and -Breaking Reactions at Sulfur(IV): Sulfoxides, Sulfonium Salts, Sulfur Ylides, and Sulfinate Salts. - Abstract - Europe PMC
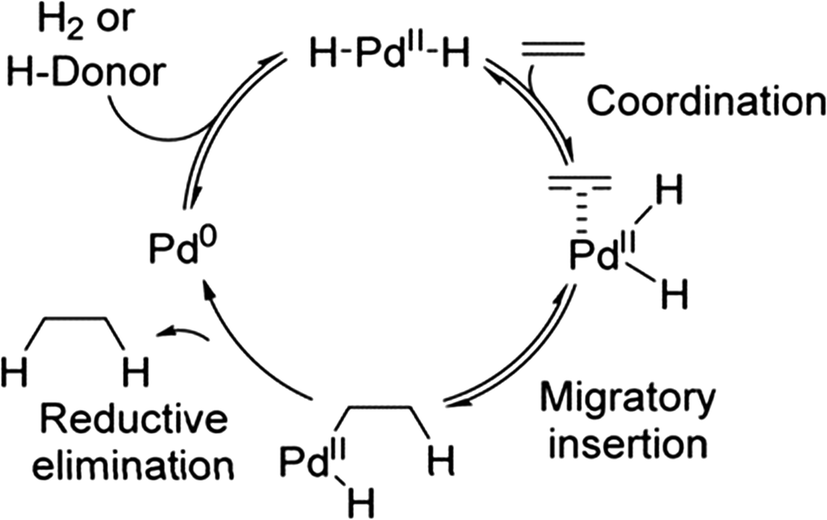
Towards high-performance heterogeneous palladium nanoparticle catalysts for sustainable liquid-phase reactions - Reaction Chemistry & Engineering (RSC Publishing)

Thiol Treatment Creates Selective Palladium Catalysts for Semihydrogenation of Internal Alkynes - ScienceDirect

Thiol Treatment Creates Selective Palladium Catalysts for Semihydrogenation of Internal Alkynes - ScienceDirect
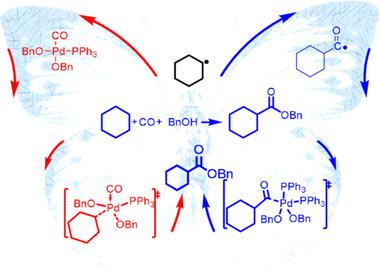
Investigating the Mechanism of Palladium‐Catalyzed Radical Oxidative C(sp3)−H Carbonylation: A DFT Study - Chem. Asian J. - X-MOL

Energy profiles for palladium-catalysed oxidative addition of PhBr to... | Download Scientific Diagram

Palladium‐Catalyzed Suzuki Coupling of N‐Acyloxazolidinones via Selective Cleavage of C–N Bonds - Jian - 2020 - European Journal of Organic Chemistry - Wiley Online Library
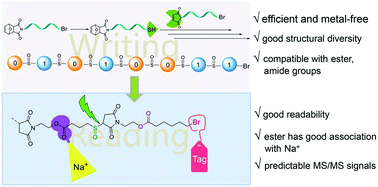
Engineering digital polymer based on thiol–maleimide Michael coupling toward effective writing and reading - Polym. Chem. - X-MOL

Energy profiles for palladium-catalysed oxidative addition of PhBr to... | Download Scientific Diagram

Thiol Treatment Creates Selective Palladium Catalysts for Semihydrogenation of Internal Alkynes - ScienceDirect