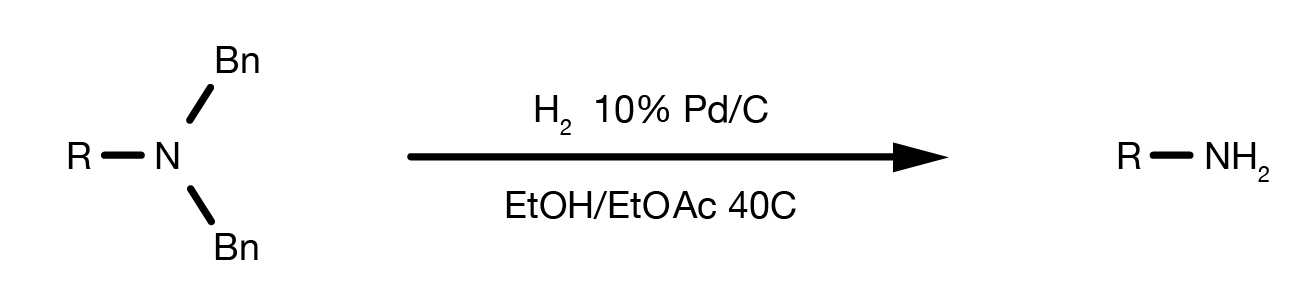
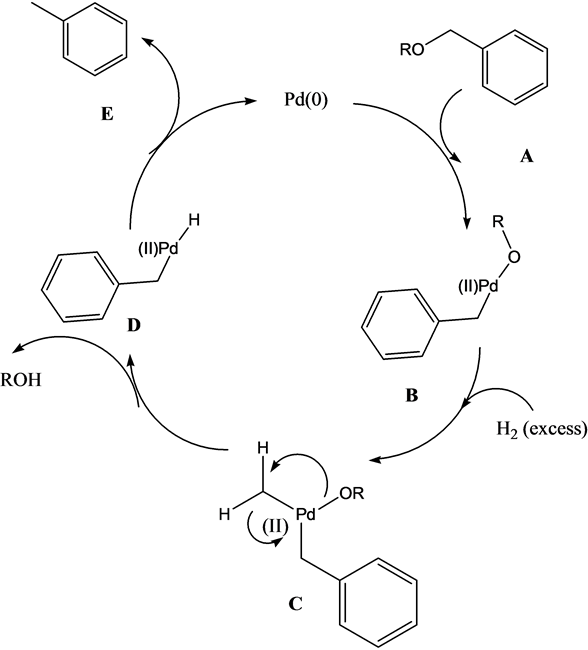Recent Progress in the Use of Pd-Catalyzed C-C Cross-Coupling Reactions in the Synthesis of Pharmaceutical Compounds

Recent Progress in the Use of Pd-Catalyzed C-C Cross-Coupling Reactions in the Synthesis of Pharmaceutical Compounds

Palladium-catalyzed reaction of tributyltin hydride. Selective and very mild deprotection of allyl and allyloxycarbonyl derivatives of amino-acids

PDF) A concise Pd catalyzed cross coupling reaction along with deprotection for the synthesis of a new series of pyrimidine derivatives

Recent Progress in the Use of Pd-Catalyzed C-C Cross-Coupling Reactions in the Synthesis of Pharmaceutical Compounds

Intracellular Deprotection Reactions Mediated by Palladium Complexes Equipped with Designed Phosphine Ligands. - Abstract - Europe PMC

Palladium-triggered deprotection chemistry for protein activation in living cells | Nature Chemistry
A Mechanistic Study of Direct Activation of Allylic Alcohols in Palladium Catalyzed Amination Reactions

Recent Progress in the Use of Pd-Catalyzed C-C Cross-Coupling Reactions in the Synthesis of Pharmaceutical Compounds

Mild Palladium‐Catalyzed Cyanation of Unprotected 2‐Iodoglycals in Aqueous Media as Versatile Tool to Access Diverse C2‐Glycoanalogues - Malinowski - 2020 - Advanced Synthesis & Catalysis - Wiley Online Library
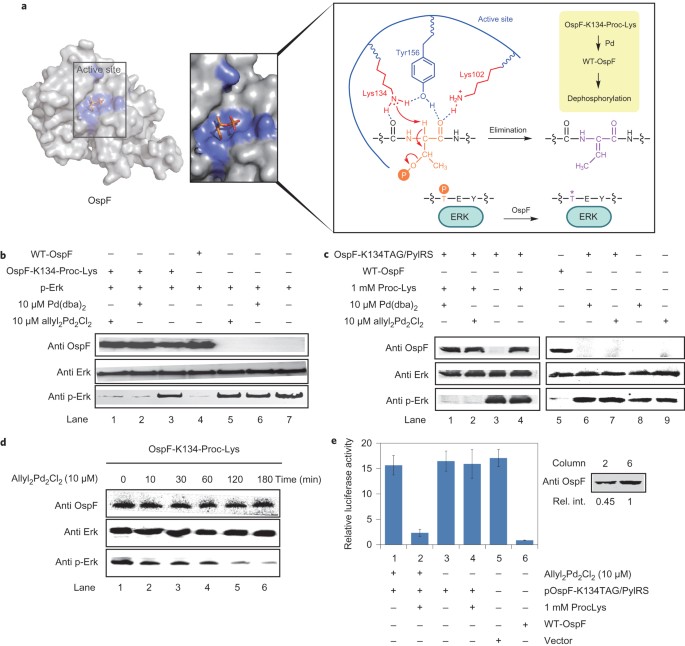
Palladium-triggered deprotection chemistry for protein activation in living cells | Nature Chemistry
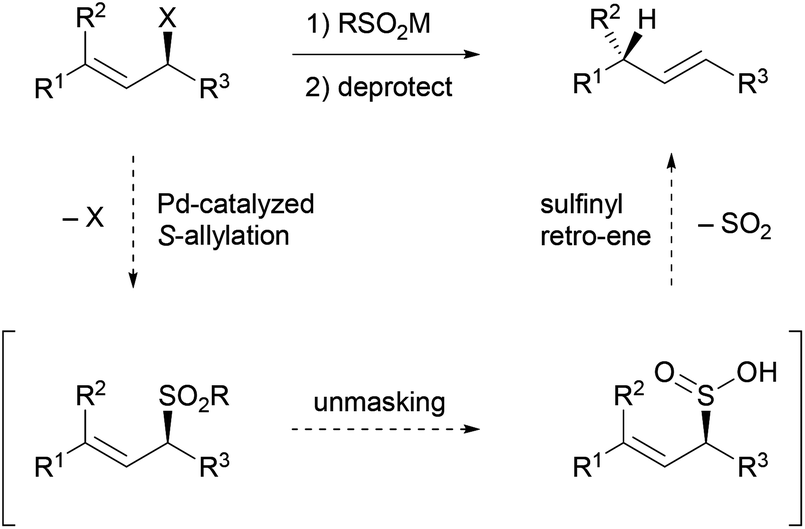
Stereoselective allylic reduction via one-pot palladium-catalyzed allylic sulfonation and sulfinyl retro-ene reactions - Organic Chemistry Frontiers (RSC Publishing)
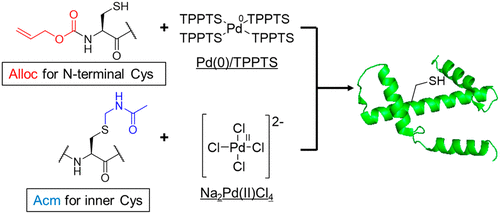
Chemical Synthesis of Cys-Containing Protein via Chemoselective Deprotection with Different Palladium Complexes.,Organic Letters - X-MOL

Pd(II)-catalyzed deprotection of acetals and ketals containing acid sensitive functional groups - ScienceDirect
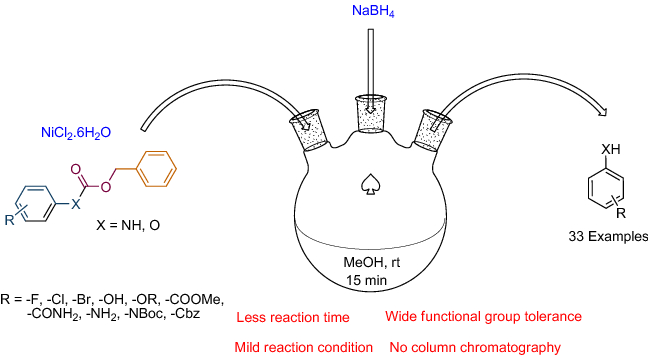
Development of a novel protocol for chemoselective deprotection of N / O -benzyloxycarbonyl (Cbz) at ambient temperature | SpringerLink

Recent Progress in the Use of Pd-Catalyzed C-C Cross-Coupling Reactions in the Synthesis of Pharmaceutical Compounds

Intracellular Deprotection Reactions Mediated by Palladium Complexes Equipped with Designed Phosphine Ligands. - Abstract - Europe PMC

Allylic protecting groups and their use in a complex environment part II: Allylic protecting groups and their removal through catalytic palladium π-allyl methodology

Facile and selective cleavage of allyl ethers, amines and esters using polymethylhydrosiloxane–ZnCl2/Pd(PPh3)4 - ScienceDirect